

It is a useful concept to understand and analyze the reactivity, polarity, color, phase of matter, magnetism, and so on. It’s very simple, just put the name of the compound or chemical formula of the compound in the blank box of the “Lewis structure generator” tool, then, click on submit, and a popup box will appear with the Lewis structure of the given compound. The geometry of molecules, which is also commonly known as molecular structure, is a 3-D structure of the entire molecule. How do you use the Lewis structure calculator tool? Lewis structure or Lewis dot structure is drawn by using 5 or 6 steps.
#Molecular geometry calculator how to#
Let’s check our full guide in – How to draw the lewis structure for any compound or ions?Īlso, check the Lewis structure for some important molecules – CO2 Lewis structure That’s all by working on the methods given above, you can calculate the lewis structure for any compound. So, an easy way to find the valence electron of atoms in the PCl 3 molecule is, just to look at the periodic group of phosphorous and chlorine atoms. We demonstrate that xmolecule is capable to calculate the whole spectrum of multiple-hole configurations at a given molecular geometry and potential energy. Steps for drawing the Lewis dot structure for PCl 3įirst of all, determine the valence electron that is available for drawing the lewis structure of PCl 3 because the lewis diagram is all about the representation of valence electrons around atoms. We can easily find out the central atom if we have a clear understanding of the electronegativity concept. Our second step will be to determine which one of the constituent atoms will act as the central atom. Let’s follow some steps and draw the lewis structure for PCl3. Step 2 We are done with calculating the total number of valence electrons of all the atoms in a given molecule. But as we know ClO4 is an ion having a negative. A three-step approach for drawing the CHCl3 molecular can be used.
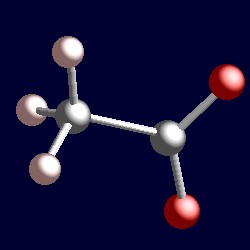
Total number of valence electrons in ClO4 71 + 64 31. Key Points To Consider When drawing The CHCl3 Molecular Geometry.
Oxygen, on the other hand, belongs to the chalcogen family of group 6, therefore having 6 electrons in its valence shell. For that, either you can use the online “Lewis structure calculator” tool or the traditional method. Chlorine is a halogen, hence it belongs to the halogen family of group 7. Complete the octet of the central atom (make multiple bonds if necessary)įor example – Let’s say we have to calculate the lewis structure for the PCl3 molecule.Complete the octet of outer atoms first.Calculate the total number of valence electrons in the molecule.For most of the compounds, the lewis structure is drawn using 5 or 6 simple steps.


 0 kommentar(er)
0 kommentar(er)
